Abstract
Background:
Despite the potential for durable disease remission seen with Chimeric Antigen Receptor (CAR-T) and T-Cell Receptor (TCR-T) therapies, their use is limited by the potential for acute toxicity from Cytokine-Release Syndrome (CRS).Across all grades, CRS has seen rates as high as 100% in some trials of patients receiving CAR-T, and up to 15% receiving TCR-T. Prior research indicates an association of hematologic abnormalities with CRS. Due to smaller average trial size and limited adoption, to date, of CAR and TCR therapies, there have been no large-scale studies to date exploring these associations with CRS severity in a wide range of patients across treatment types. This study sought to address these evidence gaps using retrospective analysis of pooled clinical trial data in Acute Lymphocytic Leukemia (ALL), using, to our knowledge, the single largest data repository of of CAR-T and TCR-T clinical patient data, with high resolution measurements across a spectrum of clinical domains.
Methods:
Eligible Phase I, II and III completed clinical trials in Acute Lymphocytic Leukemia (ALL), with patients receiving either CAR-T or TCR-T, were identified from the Medidata Enterprise Data Store, which comprises over 22,000 historical clinical trials, for de-identified retrospective aggregate analyses. Baseline characteristics, including demographics, medical history, prior treatment regimens were assessed and stratified by treatment type. Pre-trial history of hematologic conditions, such as neutropenia and anemia, were also assessed. Using Common Terminology Criteria for Adverse Events (CTCAE) 4.03, patients were assigned to categories of any CRS, mild CRS (grade 1) and moderate-to-severe CRS (2+). Hematologic function was assessed at baseline through first exposure to treatment, including counts of erythrocytes, neutrophils, eosinophils and basophils. Baseline marrow blast cell percentage was assessed as a marker of tumor burden. Univariate analyses of associations between pre-treatment baseline variables and CRS were conducted using Wilcoxon signed rank tests.
Results:
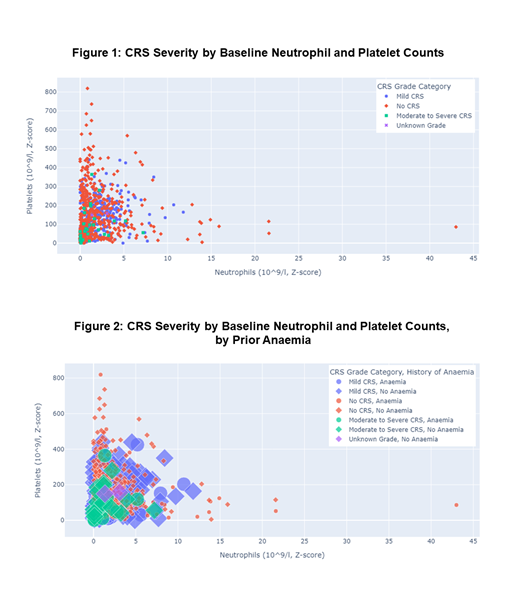
The pooled CT data contained 1,410 ALL patients, of whom over 60% were 18 year of age or greater. Baseline blood chemistries indicated 21% with anemia, 12% with thrombocytopenia, 6% neutropenic and 5.3% with elevated LDH. Although CAR-T patients accounted for 14.9% of the cohort, 47% of CRS events observed were associated with CAR-T treatment. In line with expectations from prior literature, factors associated with moderate-to-severe CRS included prior history of anemia, reduced platelet levels, low neutrophil counts, and delayed neutrophil recovery. Nearly all cases of moderate-to-severe CRS occurred in subjects exhibiting both low neutrophil and low platelet counts (Figure 1). Similar associations were seen in patients with pretreatment history of anemia (Figure 2). Consistent with literature on tumor burden and CRS, patients without CRS tended to have lower marrow blast percentages. Lymphocyte levels at baseline were far lower in patients receiving CAR-T therapy, with slower recovery than in patients receiving TCR-T. While consistent with CAR-T pre-treatment lymphodepletion, this finding was noteworthy given the association of neutropenia with CRS.
Conclusions:
Overall findings suggest patterns of routine hematologic function at baseline can potentially be used to assess risk of moderate-to-severe CRS in patients receiving CAR-T and TCR-T agents. The association with these markers could also suggest a mechanism of CRS as a function of tumor cell concentration, modified by the strength and presence of innate immunity mechanisms such as granulocytes, and potentially mediated by intermediates such as macrophages, in line with emerging literature., Further analysis may facilitate development of predictive algorithms to identify patients at greater risk for severe CRS prior to as well as shortly after treatment. This has implications for enhancing supportive care for patients receiving CAR- and TCR therapies. Additionally, a data-driven stratification of patients by risk of CRS will allow improved utilization and management of care resources.
Agarwal: Medidata Acorn AI, a Dassault Systèmes Company: Current Employment. Socolov: Medidata Acorn AI, a Dassault Systèmes Company: Current Employment. Buderi: Medidata Acorn AI, a Dassault Systèmes Company: Current Employment. Rusli: Medidata Acorn AI, a Dassault Systèmes Company: Current Employment. Bouzit: Medidata Acorn AI, a Dassault Systèmes Company: Current Employment. Talwai: Medidata Acorn AI, a Dassault Systèmes Company: Current Employment. Itzkovich: Medidata Acorn AI, a Dassault Systèmes Company: Current Employment. Galaznik: Medidata Acorn AI, a Dassault Systèmes Company: Current Employment, Current equity holder in publicly-traded company. Aptekar: Medidata Acorn AI, a Dassault Systèmes Company: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal